Real-Time Monitoring and Immediate Alerts
One of the standout features of the TVS checkweigher is its ability to monitor variations in tablet production metrics and the operational status of the tablet press. This real-time oversight is crucial for identifying potential issues before they escalate. When the system detects any abnormal production indicators, it triggers an immediate alarm, allowing operators to take swift action. Non-compliant samples are automatically segregated, ensuring that only products meeting quality standards proceed through the manufacturing process. This proactive approach minimizes the risk of defective products reaching the market, thereby safeguarding both consumer safety and the manufacturer’s reputation.
Comprehensive Data Management
In addition to its monitoring capabilities, the TVS tablet checkweigher generates a comprehensive array of data records, statistics, documents, and control charts for each detected parameter. This data management functionality is vital for compliance with stringent regulatory standards. The system adheres to the highest standards set by EU cGMP and GAMP5 regulations, as well as US FDA 21 CFR Part 11 provisions. Key features include a three-tier password access system, electronic record-keeping with electronic signatures, and audit trails. These functionalities ensure the integrity of drug manufacturing data, providing a robust framework for quality assurance.
Segment Inspection Capability
The segment inspection capability within the TVS checkweigher further enhances its functionality. When an anomaly in production indicators is identified, this feature can promptly isolate at-risk product segments. This ensures that only qualified products remain within compliant batches, while those posing quality risks are contained separately. By effectively managing at-risk products, manufacturers can significantly reduce material waste and avoid costly recalls, ultimately leading to a more efficient production process.
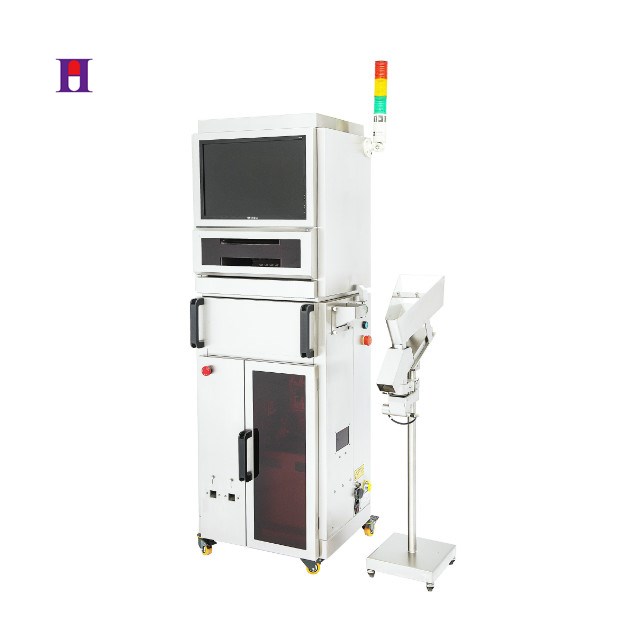
Remote Monitoring and Quality Management
The TVS checkweigher also offers remote monitoring capabilities, allowing production personnel to gain real-time insights into on-site conditions. This feature serves as an invaluable resource for quality management, enabling operators to make informed decisions based on current data. The ability to monitor production metrics remotely not only enhances operational efficiency but also empowers teams to respond quickly to any emerging issues.
Empowering Operators with SPC Principles
As a robust quality control system, the TVS checkweigher is designed specifically for managing tablet production processes. It effectively identifies issues early on, empowering operators with rapid troubleshooting tools aligned with Statistical Process Control (SPC) principles. By facilitating timely problem detection and resolution, the checkweigher helps uphold product integrity and ensures that manufacturing processes remain compliant with industry standards.
Conclusion
In conclusion, the TVS multifunctional tablet checkweigher plays a pivotal role in minimizing the scope of risk products and significantly reducing material waste in pharmaceutical manufacturing. Through real-time monitoring, comprehensive data management, and advanced segment inspection capabilities, it empowers manufacturers to maintain high-quality standards while optimizing production efficiency. As the pharmaceutical industry continues to evolve, investing in such advanced technologies will be essential for ensuring product safety, compliance, and sustainability. The TVS checkweigher not only enhances quality control but also serves as a cornerstone for responsible manufacturing practices in the pharmaceutical sector.
Send your message to us:
Post time: Oct-24-2024